
Where k is the Boltzmann constant equal to 1.38062 x10^(−23) joule/kelvin.Īnother definition, is the statistical definition developed by Ludwig Boltzmann in 1870s. If Pi is the possibility of a micro-state i, then the entropy of the system can be expressed by The latter can be defining from the probability distribution of its micro-states which demostrates, all molecular details about the system such as the position and the velocity of every molecule.

The meaning of what he says is that the entropy shows the uncertainly about the state of a system. In the 1870s the term entropy is given by J. Also just as the first law of thermodynamics leads to the definition of energy as a property of a system, so the second law, in the form of Clausius inequality, leads to the definition of a new property of fundamental importance. The word entropy has been taken from the Greek word ‘τροπη’ which means transformation.
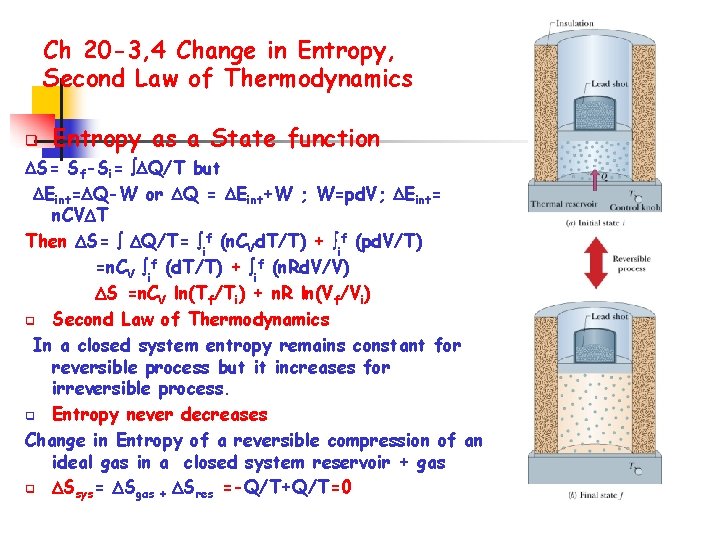
The first definition used by the German physicist Rodolf Julius Clausius in the 1850s and 1860s, he did that to state the second law of thermodynamics. The term entropy has some related definitions. Also It will give a historical overview of the term entropy and it will give some examples which are taken from the daily life and with these, I will try to explain clearly the term entropy and its intention not only in the context of the second law and also its results in our daily life. The paper examines, explain clearly, rigorously the term entropy, then discuss and evaluate its meaning in the context of the second law of thermodynamics.


 0 kommentar(er)
0 kommentar(er)
